The discovery addresses a fundamental biological puzzle: how mammals maintain energy balance during food scarcity. When facing starvation, animals like hibernators enter torpor - a suspended state marked by dramatically lowered metabolic rate, body temperature, and heart rate. While this phenomenon has significant implications for critical care medicine, organ transplantation, and space exploration, the precise neural mechanisms coordinating these physiological changes remained elusive until now.
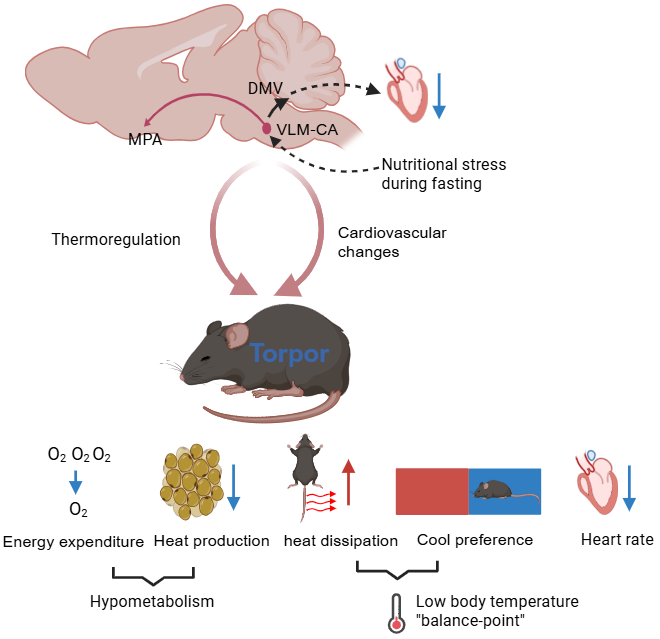
The team's experiments demonstrated that during early fasting, activity surges in specific VLM-CA neurons within mice. When researchers chemically silenced these neurons, fasting mice lost their ability to enter torpor. Conversely, activating these neurons in fed mice triggered a cascade of torpor-like effects within minutes: heart rate plummeted from 730±19 beats per minute to just 345±26 bpm, accompanied by irregular heart rhythms and occasional pauses. Body temperature followed this cardiac slowdown in a distinct temporal sequence, dropping several degrees after the heart rate decrease - mirroring the "heart-first" pattern seen in natural torpor. Simultaneously, metabolic rate plunged by 32%, brown fat temperature fell approximately 4°C, and physical activity virtually ceased.
Using viral tracing and light-sensitive controls, the researchers mapped two specialized pathways emanating from these neurons. The VLM-CA→DMV circuit proved responsible for the rapid heart rate decline and gradual cooling by stimulating vagus nerve centers, while the VLM-CA→MPA pathway maintained hypothermia by suppressing heat production. Crucially, administering the beta-agonist isoproterenol reversed both the slowed heart rate and reduced temperature, confirming that cardiac deceleration actively drives subsequent body cooling - a coordinated mechanism essential for sustaining torpor.
The conservation of this system emerged when examining hibernating Daurian ground squirrels (Spermophilus dauricus). These wild hibernators possess identical VLM-CA neurons that activate specifically during pre-hibernation phases each October, suggesting an evolutionarily conserved mechanism across species.
This work fundamentally shifts our understanding of metabolic suspension. By demonstrating that artificial VLM-CA neuron activation can replicate natural hibernation physiology, the findings open revolutionary possibilities for inducing therapeutic hypometabolism - potentially preserving organs for transplantation or protecting patients in critical care. Future research will explore these neurons' fine-grained circuitry across species and investigate their potential for managing energy metabolism disorders.
Professor Cheng Zhan (corresponding author) from the National Key Laboratory of Immune Response and Immunotherapy, School of Biomedical Sciences, University of Science and Technology of China and the Department of Hematology at the First Affiliated Hospital led this research. The work was co-first authored by Mingxiu Cheng (recent PhD graduate), Meiqi Wang (PhD candidate), and Liang Wang (research technician). Critical guidance was provided by collaborators including Professor Haohong Li (Zhejiang University), Professor Shiqiang Wang (Peking University), Professor Peng Cao (National Institute of Biological Sciences, Beijing), and Professor Yanchuan Shi (Garvan Institute of Medical Research, Australia). Funding support came from the National Natural Science Foundation of China, the Incubation Fund of the State Key Laboratory of Immune Response, and the National Key Research and Development Program of China (2030 Mega-Projects).
Article link:https://www.nature.com/articles/s41467-025-61179-1
Download article: Click here to download